R&D
Pipeline
 > R&D > Pipeline
> R&D > Pipeline
- Osteoporosis
- Anti-inflammation(Arthritis)
- Anti-inflammation(NASH)
- Anti-inflammation(IBD)
- Anti-cancer
The problem and market of IBD
- The problem
-
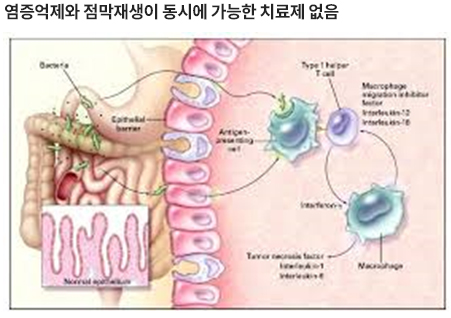
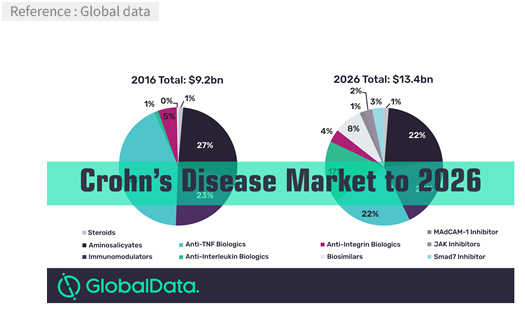
- The market
-
- The mechanism of other developed agents
-
1. Biologics – Anti TNF-α blocker
2. Aminosalicylic acid
3. Steroid
NIBEC solution for IBD
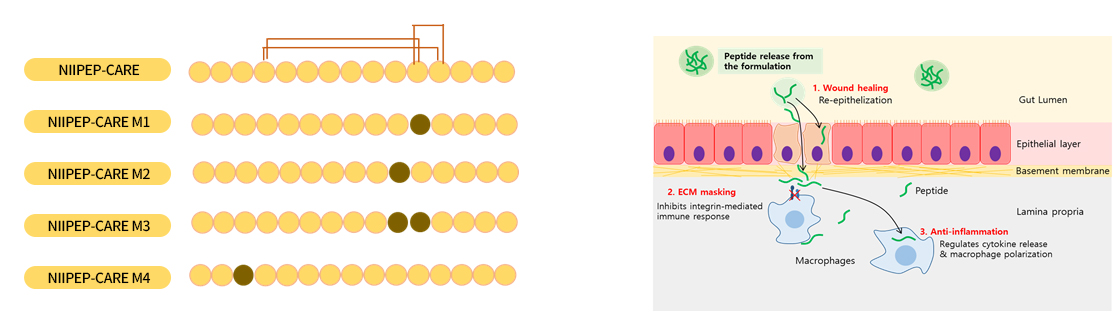
- Derivatives of NIPEP-CARE by amino acid substitution
- Dual targeting for 1) wound healing and 2) anti-inflammatory effect
- Defined target molecule (first in class) : 3 DIFFERENT IBD ASSETS DEVELOPED
- Best for immune reaction masking effect and simultaneous mucosal healing
- Recovery from inflammation invasion reflected by histology
- Current status: Preclinical efficacy, GLP TOX COMPLETED (1 asset, ready for phase 1) MOA and colon specific delivery formulation design
- Administration route: Injection, Oral colon targeting formulation
NIPEP-CARE for IBD
NIPEP-CARE improves inflammatory disease activity driven by TNBS animal model :
Treatment by the sc injection of the peptide
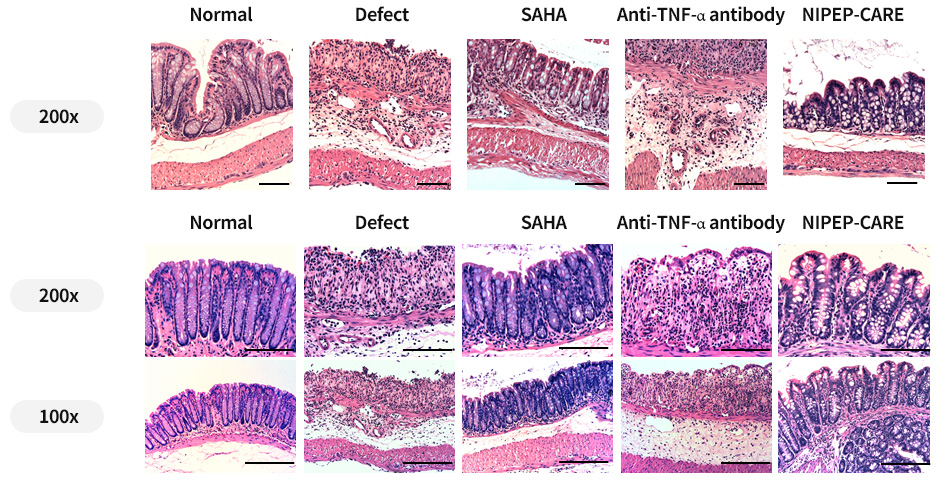
Histology of NIPEP-CARE in IBD models
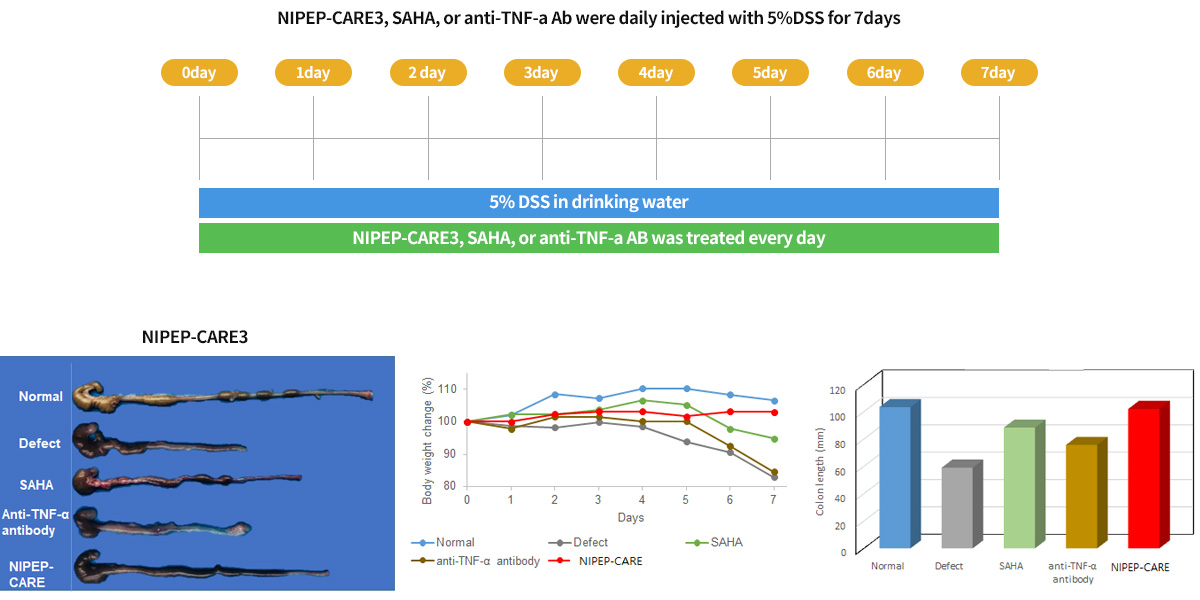
NIPEP-CARE improves inflammatory disease activity driven by DSS induced IBD model :
Treatment by the sc injection of the peptide
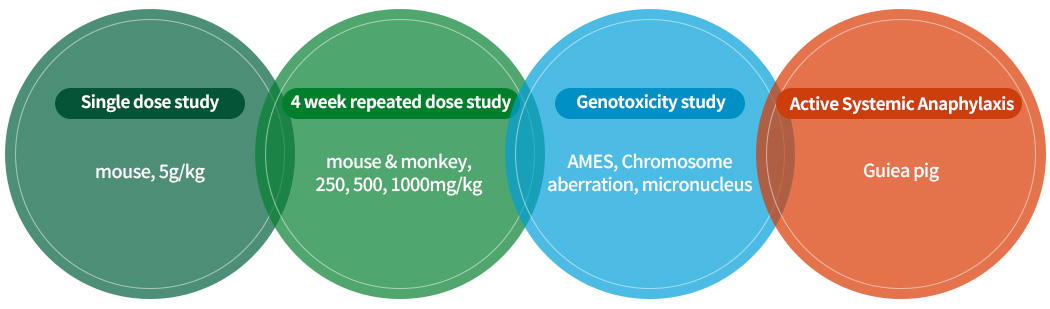
NIPEP-CARE. Non-clinical toxicity test
- Non-clinical toxicity test
- Safety pharmacology
-
Test Administration route Dose (mg/kg) Result hERG assay in vitro 300, 1000μM IC50> 1000μM Mouse neurological effect (IRWIN test) SC injection 0, 250, 500, 1000 NOAEL=1000mg/kg Monkey cardioheamodynamic effect (Telemetry) SC injection NOAEL=1000mg/kg mouse pulmonary effect (Plethysmography) SC injection NOAEL=1000mg/kg
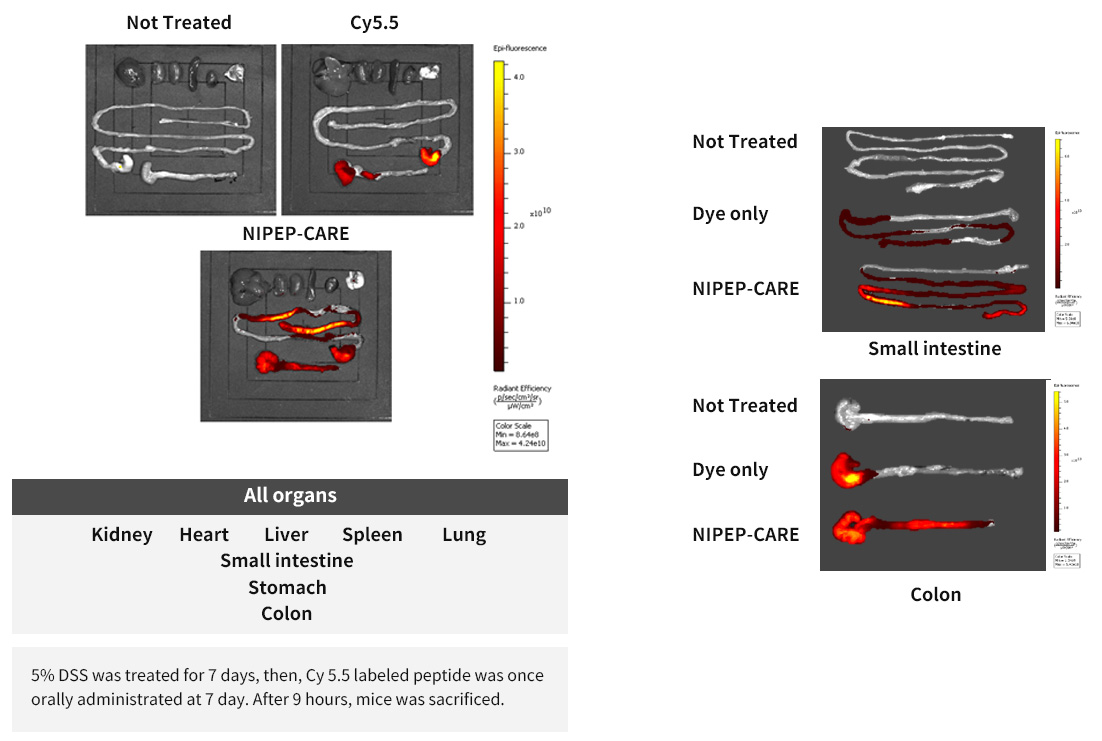
The effect of peptide ORAL administration on IBD mouse induced by 5% DSS : 7 DAY results
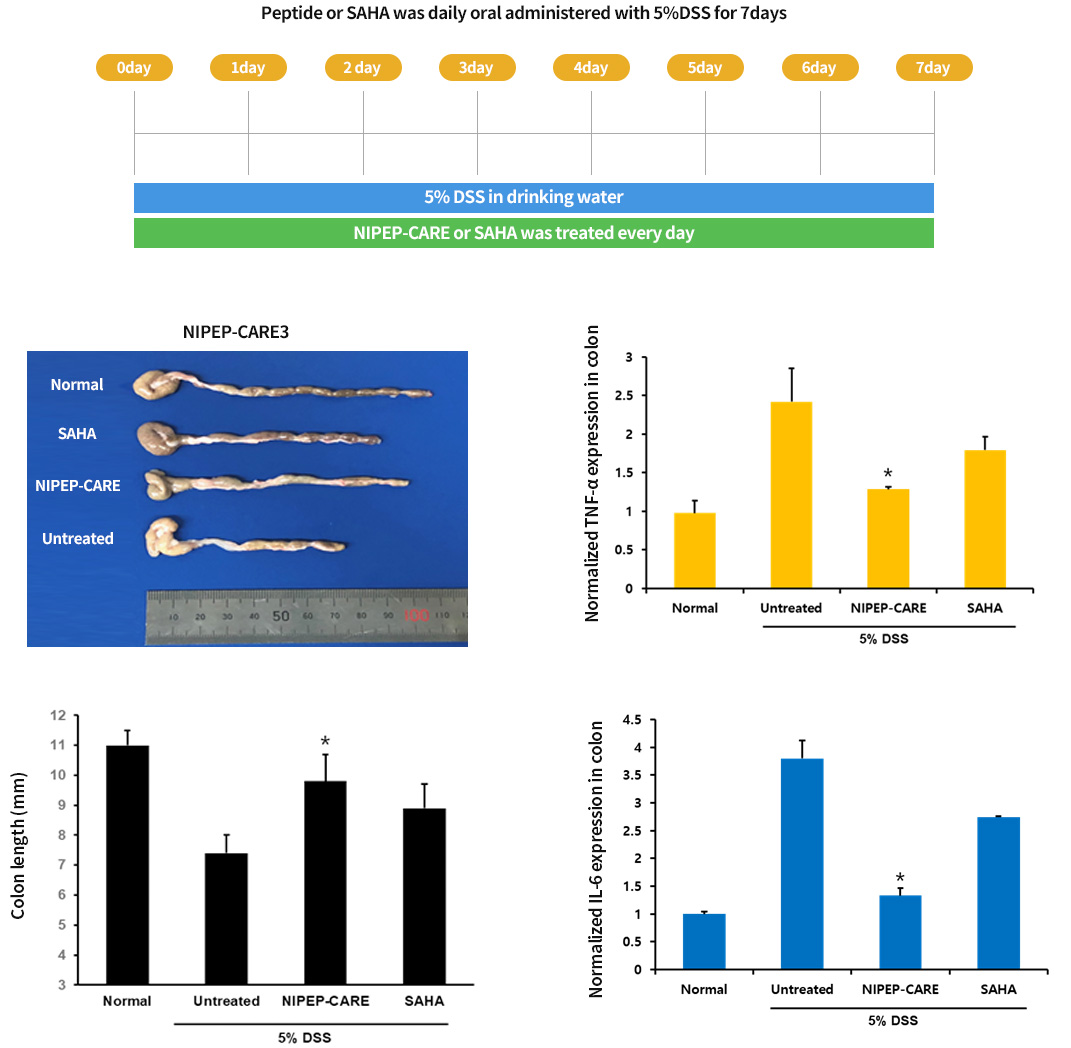
The effect of peptide ORAL administration on IBD mouse induced by 5% DSS : 7 DAY results
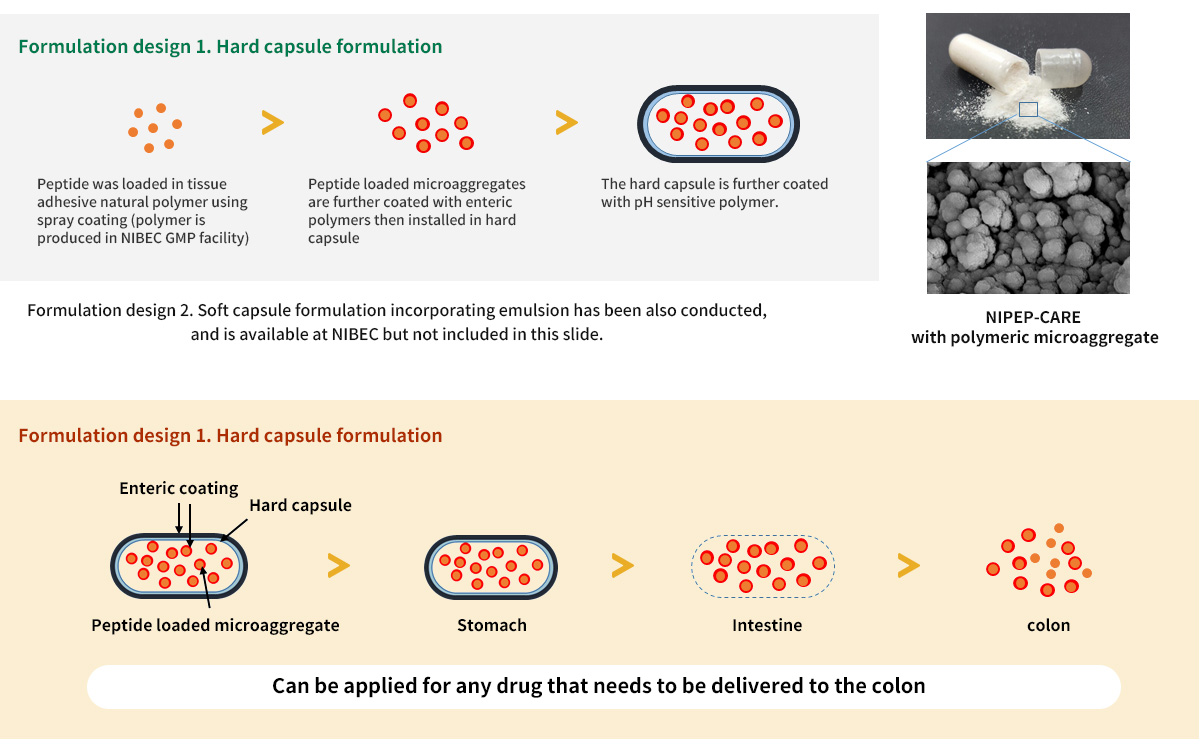
Oral formulation for colon delivery : All formulation is available at NIBEC GMP facility